Abstract
Objective
Advancing the understanding of the pathophysiology of eosinophilic oesophagitis (EoE) and other eosinophilic gastrointestinal diseases (EGIDs) has spurred research into targeted biological therapies, while the conclusive therapeutic efficacy of biologics remains uncertain. In this review, we conducted a meta-analysis of all RCTS of biologics in the treatment of EoE to evaluate their efficacy and safety and discussed their treatment of non-EoE EGIDs.
Methods
We searched the PubMed, EMBASE, Cochrane Library, and Web of Science databases. Double-blind randomized controlled trials comparing biologics with placebo in patients with EoE and non-EoE EGIDs were collected and further screened for inclusion and exclusion. The caliber of the included literature was evaluated using the Cochrane risk assessment tool findings. Data extraction and meta-analysis were conducted using RevMan 5.4 and Stata 17.0. Clinical response and histological remission were the major endpoints.
Results
Our search retrieved 3,237 articles. There were seven trials in total, comprising 792 people with EoE. Key outcomes of this meta-analysis include the following: Anti-IL-5 biologics exhibited statistically significant benefits in histological remission (RR 2.03 [CI 1.45–2.85]; p < 0.0001) compared to the placebo, but there was no significant difference in symptom relief (RR 1.06 [CI 0.88 to 1.28]; p = 0.53); anti-IL-4/13 biologics had significant effects on histologic improvement (RR 10.48 [CI 5.54–19.82]; p < 0.00001) and symptom related score reduction (RR 1.44 [CI 1.08–1.93]; p = 0.01), with a better outcome for endoscopic remission than with placebo (SMD–1.06 [CI–1.26–0.86], p < 0.00001); no statistically significant differences in adverse effects were observed between the intervention and control groups.
Conclusions
Our findings suggest that the biologics currently being investigated are considered safe and effective treatments for EoE, while their efficiency varies. However, the discussion of biologics in non-pharyngitis EGID is hampered by a lack of research, necessitating more research in high-quality trials.
Keywords: Eosinophilic esophagitis, eosinophil gastrointestinal diseases, biologic therapies, meta-analysis
KEY MESSAGES
We performed a meta-analysis using high-quality randomized controlled trials to confirm the clinical efficacy and safety of biologic therapies.
The significant superiority of biologic therapies over placebo controls and the high safety profile of biologics confirm their value for clinical use and dissemination.
At present, research results on biologics for the treatment of non-eoe EGID are still lacking and deserve to be confirmed by further studies.
1. Introduction
Eosinophilic gastrointestinal diseases (EGIDs) constitute a collection of gastrointestinal disorders distinguished by tissue eosinophilia and the manifestation of symptoms indicative of oesophageal or gastrointestinal dysfunction. According to the location of the eosinophilic infiltrate, EGIDs are classified as eosinophilic oesophagitis (EoE), gastritis (EoG), enterocolitis (EoN), and colitis (EoC) [1,2]. Clinical symptoms of EGIDs vary depending on the disease location, extent, and depth in the gastrointestinal system [3]. EoE, as the most common and extensively studied type among all EGIDs, boasts a prevalence of 42.2 cases per 100,000 adults and 34 cases per 100,000 children [4,5]. Patients suffering from EoE may present with heartburn/chest pain, dysphagia, nausea, or food impaction [6,7]. Unlike EoE, the symptoms of non-EoE EGIDs are much less specific and difficult to distinguish from other GI disorders [8]. While non-EoE EGIDs are far less common, a population-based study in the US found that the prevalence of EoG plus EoN was 5.1 per 100,000 people, and the prevalence of EoC was only 2.1 per 100,000 people [9]. A significant proportion of patients with EGIDs will have multiple sites of involvement [10]. However, the prevalence of EoE and other non-EoE EGIDs might be underestimated due to poor awareness and diagnostic delays [11].
The pathophysiological mechanisms of the various types of EGIDs are not completely known. The type 2 (T2) inflammatory response seen in EoE is similar to that seen in atopic illnesses like asthma, where elevated amounts of the characteristic T2 cytokines, IL-4, IL-5, and IL-13, are released [12]. These cytokines lead to an increase in eosinophil count, which may result in movement disorders and abnormalities in the esophageal epithelial barrier [13,14]. In contrast, non-EoE EGIDs are rarer than EoE and thus less well understood [15]. A similar T2 signature in EoE has been identified in EoG and EoN, but whether EoE and all non-EoE EGIDs share a common etiology is unclear [16].
The most prevalent therapies in EoE include pharmacologic measures, diet modification, and oesophageal dilatation for complications [17]. However, numerous patients with EoE did not attain histological remission despite the administration of corticosteroids or proton-pump inhibitors, and pharmacologic or dietary therapies have no proven benefit in non-EoE EGIDs as well [18]. With the expanding understanding of the pathogenesis of EoE and other non-EoE EGIDs, more and more biologic modulators were explored on them which exert therapeutic effects by targeting potential pathways [12]. Biologics that target Type 2 cytokines such as IL-5 (mepolizumab, reslizumab), IL-4/IL-13 (dupilumab), or IL-13 (dactrekumab, cendakimab) and that target Sialic acid-binding immunoglobulin-like lectin 8 (Siglec-8) receptor on eosinophils/mast cells have shown clinical symptom relief or histologic improvement in patients with EGIDs [19,20]. The therapeutic efficacy of biologics for EoE and other non-EoE EGIDs is still under debate. Therefore, we aim to assess the efficacy and safety of biologics compared to a placebo in the treatment of EoE patients by performing a comprehensive meta-analysis of existing high-quality, double-blind randomized controlled trials (RCTs).
2. Methods
PRISMA guidelines were followed in performing this meta-analysis (PRISMA Checklist is shown in Table S1), which was submitted to the International Prospective Register of Systematic Review (PROSPERO: No. CRD42023440744) [21].
Two reviewers independently searched the four databases of PubMed, Embase, Web of Science, and Cochrane Library for relevant literature. We also searched ClinicalTrials.gov and the European Medicines Agency, for ongoing or unpublished trials. The systematic literature search was conducted on February 11, 2024. No restrictions on language or document type were put in place. We have shown the detailed search strategies in the supplemental information (Table S2).
Inclusion criteria for this study included the following: (1) randomized controlled trials that compared outcomes between the biological therapies group and placebo group in EoE and other non-EoE EGIDs patients; (2) no age, sex, or regional limits; (3) published as complete articles with data that can be extracted; and (4) no language restrictions. Among the excluded were the following: (1) case reports/series, nonoriginal studies (letters, reviews, editorials); (2) animal or in vitro studies; (3) studies with no access to full-text content, pure abstracts of papers; (4) studies without available data can be extracted; (5) noncomparative studies; and (6) studies with duplicate and irrelevant data.
The literature review, data extraction, and quality evaluation were carried out separately by two reviewers. They used a standardized data extraction sheet to excavate pertinent data from each study that was qualified and then cross-checked the outcomes. A third reviewer was present for discussion if there were any discrepancies. This review was carried out following the Cochrane guidelines. The following information was systematically extracted from the data using a standardized data collection table: (1) generic information – comprising title, authors, country, publication year, and study design; (2) baseline characteristics – number of participants, age, sex, course of the disease, intervention facilities for the experimental and control groups, route of administration, duration of treatment/follow-up, and histologic and symptomatic response criteria; (3) outcomes – histologic, symptomatic and endoscopic improvement; and (4) adverse events.
Although we have integrated the main outcomes of the various studies, the definitions of histological improvement and symptom relief used in different trials are somewhat heterogeneous, for example, four RCTs defined histological response as <6 eosinophils/HPF, one as ≤6 eosinophils/HPF, one as <5 eosinophils/HPF, while another defined histological response as a 75% reduction in eosinophil counts in both proximal and distal esophageal biopsy specimens. Similarly, the scoring systems used for symptom relief differed, Hirano et al. [22] used the SDI score, Rothenberg et al. [23] used the MDQ questionnaire, and Dellon et al. [24] chose the EEsAI score, among others, which are specifically noted in Table 1.
Table 1.
Baseline characteristics.
| Author, year | Design | Duration | Population Age | Diagnosis and Diagnostic Criteria | Intervention | Histologic Response Criteria | Symptomatic Response Criteria | Results |
|---|---|---|---|---|---|---|---|---|
| Straumann et al., 2010 | RCT | 13 wk | ≥ 18y | EoE >20 eosinophils per HPF, at least one episode of dysphagia per wk in the previous 4 wk |
Mepolizumab (n = 5); Placebo (n = 6) | <5 eosinophils per HPF |
Presence of clinical symptoms | Symptomatic response in 2 of 5 patients in the intervention group and 1 of 6 patients in control group |
| Spergel et al., 2012 | RCT, multicenter |
15 wk | 5- 18y | EoE ≥24 eosinophils per HPF in at least 1 biopsy specimen |
Reslizumab 1, 2, 3mg/kg at wk 0, 4, 8, 12 (n1 = 55, n2 = 57, n3 = 57); Placebo (n = 57) | Responders: Reduce in peak esophageal eosinophil counts from baseline to the end of treatment | Presence of clinical symptoms with physician’s eosinophilic esophagitis global assessment scores |
Histologic response in 104 of 129 patients in the intervention group and 20 of 46 patients in the control group; Symptomatic response in 100 of 129 patients in the intervention group and 37 of 46 patients in the control group |
| Hirano et al., 2020 |
Phase 2 RCT, multicenter | 12 wk | 18-65y | EoE >15 eosinophils per HPF despite 8 wk of high-dose PPI therapy |
Dupilumab 300 mg weekly (n = 23); Placebo (n = 24) | <6 eosinophils per HPF |
Decrease of 3 points on the SDI from baseline to the end of therapy | Histologic response in 18 of 23 patients in the intervention group and 0 of 24 patients in the control group; Symptomatic response in 9 of 23 patients in the intervention group and 3 of 24 patients in the control group |
| Rothenberg et al., 2015 | RCT, multicenter |
21 wk | 18-50y | EoE ≥24 eosinophils per HPF |
QAX576 6mg/kg at wk 0, 4, 8 (n = 17); Placebo (n = 8) | 75% reduction in peak esophageal eosinophil counts in the proximal and distal esophageal biopsy specimens | Presence of clinical symptoms with MDQ | Histologic response in 7 of 17 patients in the intervention group and 1 of 8 patients in the control group; Symptomatic response in 10 of 17 patients in intervention group and 4 of 8 patients in control group |
| Dellon et al., 2022 |
Phase 3 RCT, multicenter | 24 | ≥ 12y | EoE ≥15 eosinophils per HPF despite 8 wk of high-dose PPI therapy |
PART A: Dupilumab 300mg weekly (n = 42); Placebo (n = 39) PART B: Dupilumab 300mg weekly (n = 80); Dupilumab 300mg every 2 wk (n = 81); Placebo (n = 79) |
<6 eosinophils per HPF |
Presence of clinical symptoms with the DSQ score | PART A: Histologic response in 25 of 42 patients in the intervention group and 2 of 39 patients in the control group; PART B: Histologic response in 96 of 161 patients in the intervention group and 5 of 79 patients in the control group |
| Hirano et al., 2019 |
Phase 2 RCT, multicenter | 16 wk | 18-65y | EoE ≥15 eosinophils per HPF |
RPC4046 180 mg weekly (n = 31); 360 mg weekly (n = 34); Placebo (n = 34) | <6 eosinophils per HPF |
Presence of clinical symptoms with mean clinician’s global assessment of disease severity score | Histologic response in 15 of 65 patients in the intervention group and 0 of 34 patients in the control group; Symptomatic response in 50 of 65 patients in the intervention group and 20 of 34 patients in the control group |
| Dellon et al., 2023 |
RCT, multicenter |
12 | 16-75y | EoE ≥15 eosinophils per HPF, >3 episodes over 2 weeks, and EEsAI Score ≥27 |
Mepolizumab 300mg, monthly (n = 31); Placebo (n = 33) | ≤6 eosinophils per HPF |
Presence of clinical symptoms with ≥20 decrease in EEsAI score |
Histologic response in 10 of 13 patients in the intervention group and 1 of 13 patients in the control group Symptomatic response in 50 of 65 patients in the intervention group and 20 of 34 patients in control group |
Abbreviations: HPF: high-powered field; SDI: Straumann Dysphagia Instrument; MDQ: Mayo Dysphagia Questionnaire; DSQ: Dysphagia Symptom Questionnaire; SODA: Severity of Dyspepsia Assessment; PROMIS: Patient-Reported Outcome Measurement Information System; EEsAI: Eosinophilic Esophagitis Activity Index.
We performed a rigorous examination for quality assessment and bias risk of the included RCTs. The evaluation content included the following aspects: selection bias, performance bias, detection bias, attrition bias, reporting bias, and other biases. All research findings were evaluated according to these standards using the terms ‘low risk’, ‘unclear risk’, and ‘high risk’.
Data analysis of the study was carried out by Review Manager 5.4 and STATA 17.0. Dichotomous outcomes were reported as risk ratios (RR) with 95% CI, while continuous outcomes were revealed using the standardized mean difference (MD, SMD) with 95% CI. We used I2 values to test the statistical heterogeneity, and the fixed effect model was chosen if p ≥ 0.1 and I2≤50% (statistical heterogeneity was considered to be appropriate). Subgroup analysis and sensitivity analysis were adopted to investigate the source of heterogeneity if p < 0.1 and I2>50% (statistical heterogeneity exists between the results). When it was impossible to identify the cause of heterogeneity, the random effect model was used for data analysis. The study’s publication bias was depicted using Harbord’s modified test and modified Galbraith plots. Harbord’s modified test revealed a significant difference with p < 0.05.
If we encountered incomplete data in some studies, we endeavored to contact the original researchers to make up for the missing data wherever possible.
3. Results
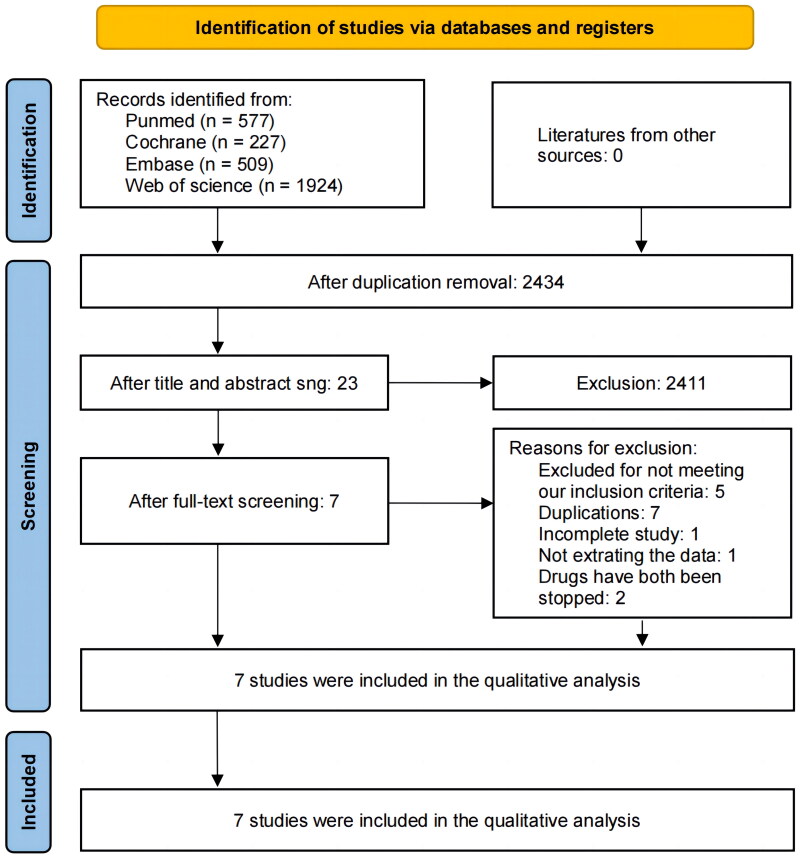
A total of 3,237 records were retrieved in our initial search, and 784 documents were removed as duplicates, leaving 2,434 records. A total of 2,411 records were disqualified after titles or abstracts were examined. A total of 23 studies were identified; 16 were ultimately eliminated because they failed to match the inclusion criteria (Table S3). One of the studies (Dellon et, al. 2022) consisted of two parts, A and B, for the sake of data extraction and analysis, and we considered them as two different trials. The flowchart of the literature screening procedure is depicted in Figure 1. These studies were all reported in English. Our meta-analysis included randomized controlled trials published up to February 11, 2024 [22–28], involving 792 patients with an age range of 5 to 75 years old, all the patients had been diagnosed with EoE. Participants in these trials were administered different biologics, 513 of whom received the treatment (experimental group) and 280 of whom received a placebo (control group), and the treatment period ranged from 12 to 24 weeks. The baseline data of each trial considered are summarized in Table 1.
Figure 1.
Flowchart of selection and screening of the studies [21].
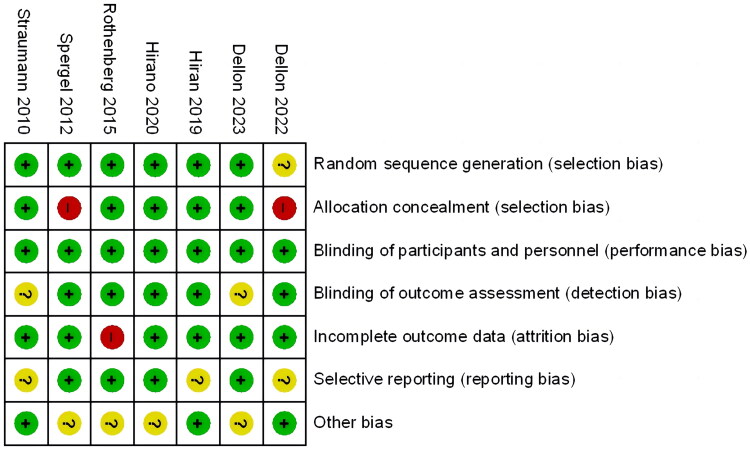
The methodological quality items of the included studies as determined by the Cochrane Risk of Bias Assessment Tool are shown in Figure 2. Because each trial was double-blinded, performance bias was assessed as low for all trials. Only one study was evaluated as having an uncertain risk for this item since it did not specify the specific method of randomized sequence generation. High risk was assigned to two studies for this item because they failed to indicate allocation concealment. In addition, two studies were given a rating of questionable risk of detection bias due to ambiguous result assessment blinding techniques. One study’s attrition bias risk was graded as uncertain because there was insufficient data on the baseline and outcome variables. Three studies were classified as having an unknown risk of reporting bias because they lacked precision in disease diagnostic criteria and effectiveness criteria.
Figure 2.
Risk of bias summary.
3.1. Histological remission
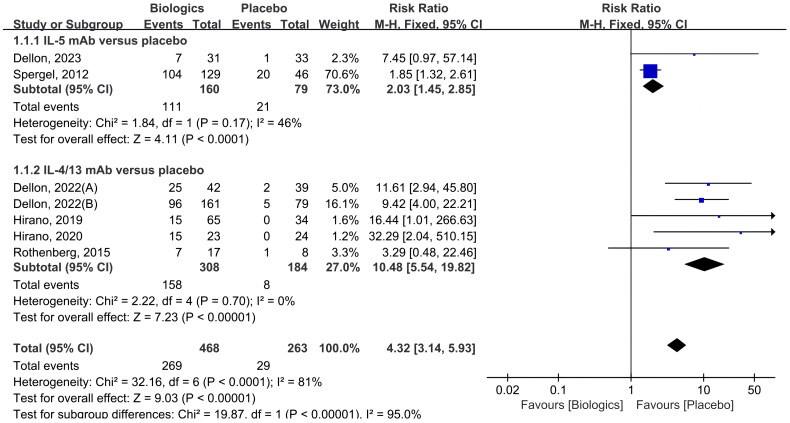
Seven RCTs included reporting of histological remission (as shown in Figure 3) [22–24,26–28], and approximately 57.5% (269 of 468) of the patients had histological remission compared with 11.0% (29 of 263) of patients in the placebo group, indicating that biologics were significantly more effective than placebo in histological remission (RR 4.32 [CI 3.14–5.93]; p < 0.00001).
Figure 3.
Biologics versus placebo: histological remission.
When stratified by the type of biologics, two studies assessed in 239 patients were identified that compared anti-IL-5 antibodies against placebo [24,26], the histological remission rate was 69.4% in the IL-5 antibody group (111 of 160) and 26.6% in the control group (21 of 79). In one RCT, the effectiveness of mepolizumab (300 mg/month; subcutaneous injection) versus placebo for EoE was assessed [24], while in another RCT, the effectiveness of Reslizumab (1, 2, 3 mg/kg at wk 0, 4, 8, 12; intravenous infusion) was examined [26]. Both therapies were superior to the biologics group in terms of histologic improvement and were statistically significant (RR 2.03 [CI 1.45–2.85]; p < 0.0001).
There were five trials with a comparison of anti-IL-4/IL-13 antibody therapy to placebo [22,23,27,28]. In this analysis, a total of 492 patients were involved. Of them, 158 of 308 (51.3%) in the biologics group and 8 of 184 (2.2%) in the placebo group experienced histological remission. Three of these trials involved using dupilumab (anti-IL-4/IL-13 antibody) as the intervention drug [22,28], one trial involved QAX576/dactrekumab (anti-IL-13 antibody) [23], and one trial involved RPC4046/cendakimab (anti-IL-13 antibody) [27]. The difference was statistically significant (RR 10.48 [CI 5.54–19.82]; p < 0.00001).
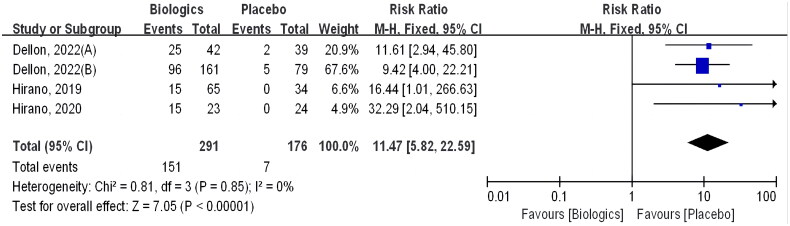
Additionally, we have summarized the studies that have set the standard for measuring efficacy in terms of <6 eos/hpf (as shown in Figure 4), all of which are about Anti-IL-4/IL-13 antibody, with three studies being about Dupilumab and one being about RPC4046. Histologic improvement was also statistically greater in the biologic group than in the placebo group (RR 11.47 [CI 5.82–22.59]; p < 0.00001).
Figure 4.
Biologics versus placebo: histological remission (<6 eosinophils per HPF).
3.2. Symptomatic response
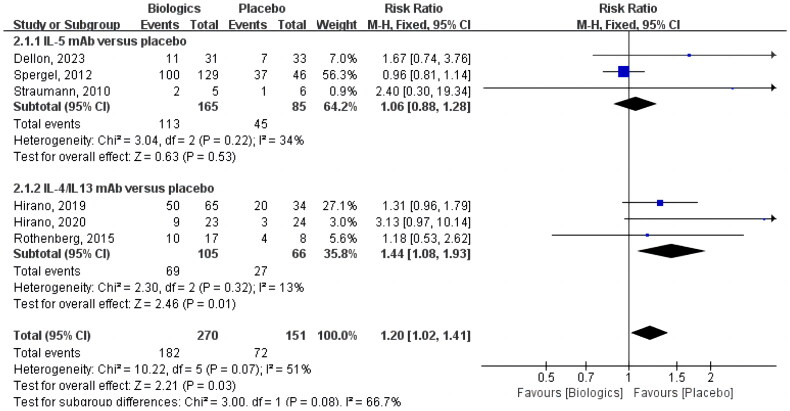
Six studies included reporting of the symptomatic response (as shown in Figure 5), which was 67.4% (182 of 270) in the biologics group and 47.7% (72 of 151) in the placebo group (RR 1.20 [CI 1.02–1.41]; p = 0.03). Although these studies applied different scores, the primary focus was on the treatment of symptoms linked to swallowing problems.
Figure 5.
Biologics versus placebo: symptomatic response.
Three trials were recognized in the subgroup analysis by biological type that used anti-IL-5 monoclonal antibodies as an intervention [24–26]. The symptomatic response rate was 68.5% (113 of 165) in the IL-5 antibody group and 52.9% (45 of 85) in the control group. In two of the trials, the effective rate of mepolizumab versus placebo for EoE was compared [24,25], while in another trial, reslizumab was used as the intervention drug [26]. However, none of the three studies produced results that were statistically significant in terms of symptom improvement (RR 1.06 [CI 0.88–1.28]; p = 0.53).
In three studies totaling 171 patients, anti-IL-4/IL-13 monoclonal antibody response versus placebo in patients with EoE was analyzed [22,23,27]. Compared to 27 of 66 (40.9%) in the control group, 69 of 105 (65.7%) participants in the intervention group experienced a symptomatic response. Anti-IL-4/IL-13 antibody dupilumab was used as the intervention therapy in one of the trials [22], while anti-IL-13 antibody (QAX576/dactrekumab and RPC4046/cendakimab) were used in the other two trials [23,27]. Overall, this result of the analysis reached statistical significance (RR 1.44 [CI 1.08–1.93]; p = 0.01).
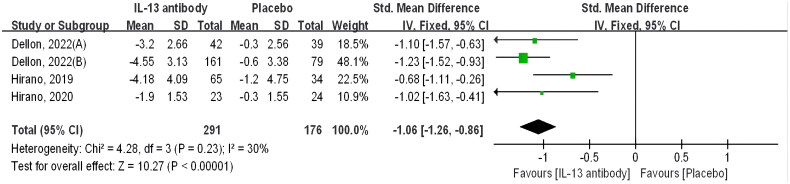
3.3. Endoscopic changes
Four trials included reporting of endoscopic assessment outcomes, all four trials were evaluated with the Eosinophilic Oesophagitis Endoscopic Reference Score [EoE-EREFS]. And all of these RCTs focused on Anti-IL-4/IL-13 antibodies; specifically, three studies investigated Dupilumab while one study explored RPC4046. According to this analysis, the intervention group given biologics demonstrated a more pronounced endoscopic improvement compared to the placebo group [SMD −1.06 [CI −1.26–0.86], p < 0.00001]. Statistics demonstrated the significance of the difference (Figure 6).
Figure 6.
Biologics versus placebo: endoscopic changes.
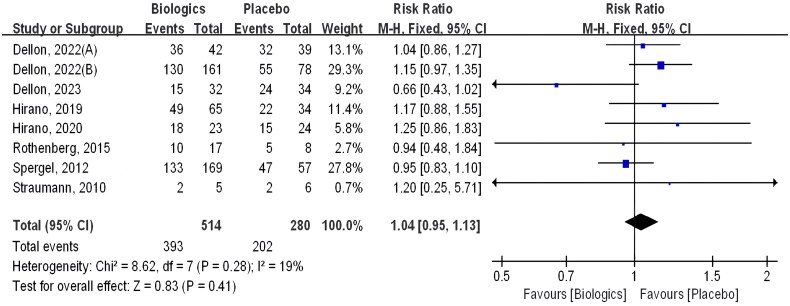
3.4. Adverse effects
Adverse effects were examined in all eight trials, and they included but were not limited to infusion-related reactions, upper respiratory tract infection, headache, nausea, vomiting, diarrhea, abdominal pain, cough, dermatitis, and arthralgia. Adverse event rates were 76.5% in the biologics group (393 of 514) and 72.1% in the control group (202 of 280). There was no significant difference between them (RR 1.04 [CI 0.95–1.13]; p = 0.41) (Figure 7).
Figure 7.
Biologics versus placebo: Adverse events.
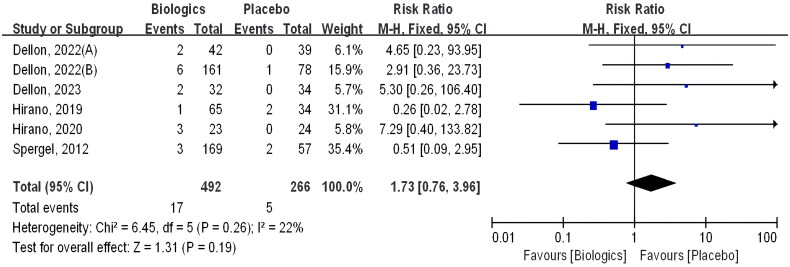
Six of these trials included reporting of serious adverse events, which involved a total of 758 patients; 17 of 492 (3.5%) in the intervention group had serious adverse events, compared with 5 of 266 (1.9%) in the control group, there was no statistical significance for this outcome (RR 1.73 [CI 0.76–3.96]; p = 0.19) (Figure 8).
Figure 8.
Biologics versus placebo: serious adverse events.
3.5. Publication bias and sensitivity analyses
Publication bias was tested for histological remission (n = 7) and symptomatic response (n = 6). Harbord’s modified test yielded a histological remission of p = 0.389, indicating no publication bias. Similarly, Harbord’s test suggested no significant publication bias in symptomatic response (p = 0.680). The modified Galbraith plots involving the included studies on symptomatic response and histological remission were drawn with Stata 17.0 (as shown in Figures S1–S2).
We performed sensitivity analyses by systematically excluding individual studies, and the results remained consistent throughout this process. This confirmed the stability of this meta-analysis outcomes (as shown in Figures S3–S4).
4. Discussion
This study comprehensively reviewed published double-blind randomized controlled trials investigating the efficacy and safety of biologics in treating EoE and non-EoE EGIDs. The primary objective was to assess the therapeutic effects of biologics and offer insights into potential advancements in this area. However, despite extensive search efforts, only seven articles focusing on EoE met the inclusion criteria. These studies encompassed interventions involving five distinct biological agents. Notably, while two trials explored biologics for non-EoE EGIDs and demonstrated some therapeutic benefits, these interventions (benralizumab and lirentelimab) have been discontinued by the manufacturer due to insufficient efficacy in the Phase III trials [29,30]. Overall, our meta-analysis revealed that biologics effectively alleviated clinical symptoms in EoE patients (RR 1.20 [CI 1.02–1.41]; p = 0.03) and induced histological improvements (RR 4.32 [CI 3.14–5.93]; p < 0.00001) compared to placebo. Although differences are observed in symptomatic improvement among various biologic classes, our study provides compelling evidence supporting biologics as a promising therapeutic avenue for EoE.
Despite EoE and other non-EoE EGIDs being diagnosed more frequently over time as a consequence of escalating incidence rates and enhanced recognition, the heterogeneity of their symptoms and the complexity of pathogenesis still make treating them a difficult task. Most drugs currently lack specific indications or approvals for use in EoE and other non-EoE EGIDs [12,31]. Primarily recommended therapies for EoE encompass PPIs, corticosteroids, dietary adjustments, or surgical intervention, while oral corticosteroids, dietary modifications, and anti-allergy medications are likewise suggested for non-EoE EGIDs. Nonetheless, the substantiating evidence for these approaches is limited due to concerns regarding efficacy gaps and potential medication-related adverse effects, and data on dietary and oral medications for long-term treatment of EoE or other non-EoE EGIDs are lacking [32–34]. To address these demands and foster innovative treatment strategies, an increasing number of clinical trials initiated in recent years to evaluate biologics for EoE or other non-EoE EGIDs are actively ongoing. Biologics were not endorsed as a treatment for EoE in the guidelines, which were published in 2017 and 2020 [33,35]. It is noteworthy that the US Food and Drug Administration (FDA) has approved dupilumab for EoE treatment in 2022 [36]. In past meta-analyses, only a very limited number of articles have presented evaluations of the efficacy of biologics for EoE, and a small subset of biologics-related RCTs have been considered [37–39]. Therefore, a thorough meta-analysis that statistically summarizes all current data on the use of biologics to treat EoE is still absent.
Biologics targeting IL-5 have been exploited and investigated in EoE and other non-EoE EGIDs, such as mepolizumab, reslizumab, and benralizumab. Mepolizumab and reslizumab bind directly to the IL-5 molecule, interfering with its attachment to IL-5Rα expressed on the eosinophil cell surface. While Benralizumab targets the eosinophilic IL-5Rα chain expressed on the granulocyte membrane, blocking IL-5 access to its receptor [40,41]. However, Benralizumab has been discontinued by its manufacturer presently. In a recent randomized controlled trial (RCT) assessing Benralizumab, it was observed that although the medication alleviates hypereosinophilia by eliminating all circulating eosinophils in EoG, patients continued to exhibit persistent signs, symptoms, and biomarkers associated with EoG [30]. Our study revealed that EoE patients’ histologic remission and endoscopic improvement were both significantly enhanced by treatment with anti-IL-5 monoclonal antibodies. Nevertheless, the same effects were not observed in symptom evaluation. One possible explanation for the disparities in histological and symptomatic responses resulting from anti-IL-5 monoclonal antibody treatment is that the RCTs we included used different criteria to assess symptomatic responses. One example is the EoE Symptom Activity Index (EEsAI) score, which focuses on the symptoms of dysphagia, whereas the physician’s eosinophilic oesophagitis global assessment scores were used for analyses that focus on the overall assessment of patient symptoms. However, there is no consensus on which scores to use to assess these patients, so studies of the different scores used were summarized. Indeed, the assertion posited by Kliewer et al. in their study presents another plausible interpretation suggesting that the pathogenesis of EGIDs may not solely hinge on eosinophilic mechanisms. Instead, it underscores a predilection for a comprehensive type 2 immune targeting approach.
Emerging clinical and experimental evidence underscores that excess IL-4 and IL-13 are central drivers in EoE [42,43]. A monoclonal antibody called dupilumab targets IL-4R and functions by recognizing and blocking the common receptor of the IL-4 and IL-13 molecules [28]. It has been endorsed to treat several eosinophilic inflammatory diseases, including EoE [14]. Additionally, the study included two anti-IL-13 monoclonal antibodies, QAX576 and RPC4046. By directly interacting with the IL-13 molecule, QAX576 prevents both the synthesis of eotaxin and IL-13 activity. RPC4046 acts by inhibiting the binding of IL-13 to the IL-13Rα receptor subunits [44]. Our analysis indicates that biologics aiming to block IL-4/IL-13 have achieved statistically significant improvements in histological, clinical, and endoscopic aspects of treating EoE. The latest research suggests that dupilumab also exhibits higher long-term efficacy in EoE [45]. Currently, no published RCT are investigating the efficacy of biologics in treating non-eosinophilic EGIDs. Only one relevant clinical trial (NCT03678545) is underway, highlighting the scarcity of research in this area.
Siglec-8, an inhibitory receptor on human eosinophils and mast cells, has manifested as a prospective therapeutic target in allergy and inflammatory diseases. There are currently two kinds of Siglec-8-targeted biologics, AK001 and AK002, in the clinical development stage [40,46,47]. AK002 is a nonfucosylated IgG1 anti-Siglec-8 monoclonal antibody. Findings from a phase II clinical trial demonstrated that lirentelimab, an anti-Siglec-8 antibody, led to decreased gastrointestinal eosinophils and alleviated symptoms in individuals with EoG and eosinophilic duodenitis (EoD) [29]. However, data from the phase III clinical trial indicated that while patients with EoE, EoG, and EoD exhibited improved histology, symptomatic relief was not observed. It is noteworthy that lirentelimab was discontinued due to insufficient clinical efficacy. Several clinical studies on AK002 are still in progress, and further follow-up studies are needed in the future.
All of the biologics included in our study exhibited a generally well-tolerated profile, with a low incidence of adverse events during treatment, most of which were mild to moderate. Notably, adverse events during therapy occurred approximately equally frequently between the biologics and control groups. The most prevalent reported adverse events included nonsevere injection site reactions, nasopharyngitis, headache, and cough. During the double-blind period, only a negligible number of patients experienced severe side effects that were almost unrelated to the treatment. Furthermore, no fatalities were reported across all studies, and vital signs and other ancillary tests of patients showed no clinically meaningful alterations. Given the chronicity of EoE, the prolonged administration of both systemic and topical corticosteroids is imperative, with substantial side effects, and withdrawal may lead to the recurrence of symptoms [48]. In this context, biological agents have demonstrated pronounced advantages.
A primary limitation of this study is the limited scope of included trials, all of which exclusively focused on EoE. Consequently, there is a notable paucity of data concerning the efficacy of biologics in treating non-EoE EGIDs. We were unable to further categorize the treated group by age range due to the small number of trials. Moreover, the heterogeneity among the articles was exacerbated by the different types and doses of biologics. Although we performed subgroup analyses based on different types of biologics, the number of studies related to each biologic was too small to conduct further dose-response studies. In addition, there was heterogeneity in the diagnosis of EoE among the included studies. While in the majority of studies, EoE was defined as having eosinophil counts of at least 15 per high-power field (HPF), a few studies used higher thresholds, such as at least 20 or 24 eosinophils per HPF. This variation could introduce selection bias by excluding patients with milder cases from the study. Finally, the criteria for outcome indicators exhibited variability across the studies, including variations in symptom scores and definitions of histological improvement. The results may be affected by greater heterogeneity.
In conclusion, our study results showed that biological therapies for EoE have proven effective in reducing eosinophil counts, alleviating clinical symptoms, mitigating endoscopic abnormalities, and maintaining a favorable safety profile. Novel biological agents are considered convenient alternatives and merit additional research, particularly given the limited efficacy of both medical and dietary treatments for EoE and other non-EoE EGIDs. Nevertheless, given the constraints of this study, the determination of the optimal dose, duration, and long-term safety of biological agents lacks substantial support from more definitive data. More high-quality trials, preferably incorporating clinical assessments with validated and standardized scoring systems, should be conducted in the future to investigate the ideal dosage of biologics as well as identify the appropriate patient population for treatment.
Supplementary Material
Acknowledgments
Thank for all investigators of the clinical trials we included.
Funding Statement
This work was supported by the Key Research and Development Projects of Shaanxi Province (S2023-YF-YBSF-1670).
Author contributions
Beibei Zeng and Doudou Jia conceptualized and designed the study, conducted the literature search, analyzed data, drafted the manuscript, and reviewed and revised the manuscript; Shengen Li and Xuna Liu reviewed literature, extracted data, evaluated quality, and revised the manuscript; Boxu Zhu, Yanqi Zhang, and Yan Zhuang designed the data collection instruments, analyzed data, and revised the manuscript; Dr Fei Dai conceptualized and designed the study, coordinated and supervised data collection, and critically reviewed the manuscript for important intellectual content. The final manuscript as submitted was approved by all authors and they agreed to accept responsibility for all aspects of this work.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Trial registry
PROSPERO; No. CRD42023440744; URL: http://www.crd.york.ac.uk/PROSPERO.
Data availability statement
The corresponding author is available with the study’s data upon reasonable request.
References
- 1.Gonsalves N. Eosinophilic gastrointestinal disorders. Clin Rev Allergy Immunol. 2019;57(2):272–285. doi: 10.1007/s12016-019-08732-1. [DOI] [PubMed] [Google Scholar]
- 2.Wright BL, Schwartz JT, Ruffner MA, et al. Eosinophilic gastrointestinal diseases make a name for themselves: a new consensus statement with updated nomenclature. J Allergy Clin Immunol. 2022;150(2):291–293. doi: 10.1016/j.jaci.2022.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walker MM, Potter M, Talley NJ.. Eosinophilic gastroenteritis and other eosinophilic gut diseases distal to the oesophagus. Lancet Gastroenterol Hepatol. 2018;3(4):271–280. doi: 10.1016/S2468-1253(18)30005-0. [DOI] [PubMed] [Google Scholar]
- 4.Dhar A, Haboubi HN, Attwood SE, et al. British Society of Gastroenterology (BSG) and British Society of Paediatric Gastroenterology, Hepatology and Nutrition (BSPGHAN) joint consensus guidelines on the diagnosis and management of eosinophilic oesophagitis in children and adults. Gut. 2022;71(8):1459–1487. doi: 10.1136/gutjnl-2022-327326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Navarro P, Arias Á, Arias-González L, et al. Systematic review with meta-analysis: the growing incidence and prevalence of eosinophilic oesophagitis in children and adults in population-based studies. Aliment Pharmacol Ther. 2019;49(9):1116–1125. doi: 10.1111/apt.15231. [DOI] [PubMed] [Google Scholar]
- 6.Meek PD, Hemstreet B.. Emerging therapies for eosinophilic esophagitis. Pharmacotherapy. 2023;43(4):338–348. doi: 10.1002/phar.2783. [DOI] [PubMed] [Google Scholar]
- 7.Koutri E, Papadopoulou A.. Eosinophilic gastrointestinal diseases in childhood. Ann Nutr Metab. 2018;73 Suppl 4(Suppl 4):18–28. doi: 10.1159/000493668. [DOI] [PubMed] [Google Scholar]
- 8.Pesek RD, Rothenberg ME.. Eosinophilic gastrointestinal disease below the belt. J Allergy Clin Immunol. 2020;145(1):87–89.e1. doi: 10.1016/j.jaci.2019.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mansoor E, Saleh MA, Cooper GS.. Prevalence of eosinophilic gastroenteritis and colitis in a population-based study, from 2012 to 2017. Clin Gastroenterol Hepatol. 2017;15(11):1733–1741. doi: 10.1016/j.cgh.2017.05.050. [DOI] [PubMed] [Google Scholar]
- 10.Jensen ET, Martin CF, Kappelman MD, et al. Prevalence of eosinophilic gastritis, gastroenteritis, and colitis: estimates from a National Administrative Database. J Pediatr Gastroenterol Nutr. 2016;62(1):36–42. doi: 10.1097/MPG.0000000000000865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rossi CM, Lenti MV, Merli S, et al. Primary eosinophilic gastrointestinal disorders and allergy: clinical and therapeutic implications. Clin Transl Allergy. 2022;12(5):e12146. doi: 10.1002/clt2.12146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hua S, Cook D, Walker MM, et al. Pharmacological treatment of eosinophilic gastrointestinal disorders. Expert Rev Clin Pharmacol. 2016;9(9):1195–1209. doi: 10.1080/17512433.2016.1190268. [DOI] [PubMed] [Google Scholar]
- 13.Katzka DA. Eosinophilic esophagitis. Ann Intern Med. 2020;172(9):Itc65–itc80. doi: 10.7326/AITC202005050. [DOI] [PubMed] [Google Scholar]
- 14.Dellon ES, Spergel JM.. Biologics in eosinophilic gastrointestinal diseases. Ann Allergy Asthma Immunol. 2023;130(1):21–27. doi: 10.1016/j.anai.2022.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shoda T, Wen T, Caldwell JM, et al. Molecular, endoscopic, histologic, and circulating biomarker-based diagnosis of eosinophilic gastritis: multi-site study. J Allergy Clin Immunol. 2020;145(1):255–269. doi: 10.1016/j.jaci.2019.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Egan M, Furuta GT.. Eosinophilic gastrointestinal diseases beyond eosinophilic esophagitis. Ann Allergy Asthma Immunol. 2018;121(2):162–167. doi: 10.1016/j.anai.2018.06.013. [DOI] [PubMed] [Google Scholar]
- 17.Rank MA, Sharaf RN, Furuta GT, et al. Technical review on the Management of Eosinophilic Esophagitis: a report from the AGA Institute and the Joint Task Force on Allergy-Immunology Practice Parameters. Gastroenterology. 2020;158(6):1789–1810.e15. doi: 10.1053/j.gastro.2020.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peterson K, Safroneeva E, Schoepfer A.. Emerging therapies for eosinophilic gastrointestinal diseases. J Allergy Clin Immunol Pract. 2021;9(9):3276–3281. doi: 10.1016/j.jaip.2021.07.031. [DOI] [PubMed] [Google Scholar]
- 19.Hirano I, Furuta GT.. Approaches and challenges to management of pediatric and adult patients with eosinophilic esophagitis. Gastroenterology. 2020;158(4):840–851. doi: 10.1053/j.gastro.2019.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dellon ES, Simon D, Wechsler ME.. Controversies in allergy: the potential role of biologics as first-line therapy in eosinophilic disorders. J Allergy Clin Immunol Pract. 2022;10(5):1169–1176. doi: 10.1016/j.jaip.2022.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hirano I, Dellon ES, Hamilton JD, et al. Efficacy of dupilumab in a phase 2 randomized trial of adults with active eosinophilic esophagitis. Gastroenterology. 2020;158(1):111–122.e10. doi: 10.1053/j.gastro.2019.09.042. [DOI] [PubMed] [Google Scholar]
- 23.Rothenberg ME, Wen T, Greenberg A, et al. Intravenous anti-IL-13 mAb QAX576 for the treatment of eosinophilic esophagitis. J Allergy Clin Immunol. 2015;135(2):500–507. doi: 10.1016/j.jaci.2014.07.049. [DOI] [PubMed] [Google Scholar]
- 24.Dellon ES, Peterson KA, Mitlyng BL, et al. Mepolizumab for treatment of adolescents and adults with eosinophilic oesophagitis: a multicentre, randomised, double-blind, placebo-controlled clinical trial. Gut. 2023;72(10):1828–1837. doi: 10.1136/gutjnl-2023-330337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Straumann A, Conus S, Grzonka P, et al. Anti-interleukin-5 antibody treatment (mepolizumab) in active eosinophilic oesophagitis: a randomised, placebo-controlled, double-blind trial. Gut. 2010;59(1):21–30. doi: 10.1136/gut.2009.178558. [DOI] [PubMed] [Google Scholar]
- 26.Spergel JM, Rothenberg ME, Collins MH, et al. Reslizumab in children and adolescents with eosinophilic esophagitis: results of a double-blind, randomized, placebo-controlled trial. J Allergy Clin Immunol. 2012;129(2):456–463.e3. 63.e1-3. doi: 10.1016/j.jaci.2011.11.044. [DOI] [PubMed] [Google Scholar]
- 27.Hirano I, Collins MH, Assouline-Dayan Y, et al. RPC4046, a monoclonal antibody against IL13, reduces histologic and endoscopic activity in patients with eosinophilic esophagitis. Gastroenterology. 2019;156(3):592–603.e10. doi: 10.1053/j.gastro.2018.10.051. [DOI] [PubMed] [Google Scholar]
- 28.Dellon ES, Rothenberg ME, Collins MH, et al. Dupilumab in adults and adolescents with eosinophilic esophagitis. N Engl J Med. 2022;387(25):2317–2330. doi: 10.1056/NEJMoa2205982. [DOI] [PubMed] [Google Scholar]
- 29.Dellon ES, Peterson KA, Murray JA, et al. Anti-siglec-8 antibody for eosinophilic gastritis and duodenitis. N Engl J Med. 2020;383(17):1624–1634. doi: 10.1056/NEJMoa2012047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kliewer KL, Murray-Petzold C, Collins MH, et al. Benralizumab for eosinophilic gastritis: a single-site, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Gastroenterol Hepatol. 2023;8(9):803–815. doi: 10.1016/S2468-1253(23)00145-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pesek RD, Reed CC, Muir AB, et al. Increasing rates of diagnosis, substantial co-occurrence, and variable treatment patterns of eosinophilic gastritis, gastroenteritis, and colitis based on 10-year data across a multicenter consortium. Am J Gastroenterol. 2019;114(6):984–994. doi: 10.14309/ajg.0000000000000228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Visaggi P, Ghisa M, Barberio B, et al. Treatment trends for eosinophilic esophagitis and the other eosinophilic gastrointestinal diseases: systematic review of clinical trials. Dig Liver Dis. 2023;55(2):208–222. doi: 10.1016/j.dld.2022.05.004. [DOI] [PubMed] [Google Scholar]
- 33.Hirano I, Chan ES, Rank MA, et al. AGA Institute and the Joint Task Force on Allergy-Immunology Practice Parameters Clinical Guidelines for the Management of Eosinophilic Esophagitis. Gastroenterology. 2020;158(6):1776–1786. doi: 10.1053/j.gastro.2020.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu X, Xiao X, Liu D, et al. A meta-analysis on randomized controlled trials of treating eosinophilic esophagitis with budesonide. Ann Med. 2022;54(1):2078–2088. doi: 10.1080/07853890.2022.2101689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lucendo AJ, Molina-Infante J, Arias Á, et al. Guidelines on eosinophilic esophagitis: evidence-based statements and recommendations for diagnosis and management in children and adults. United European Gastroenterol J. 2017;5(3):335–358. doi: 10.1177/2050640616689525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rothenberg ME. Scientific journey to the first FDA-approved drug for eosinophilic esophagitis. J Allergy Clin Immunol. 2022;150(6):1325–1332. doi: 10.1016/j.jaci.2022.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rokkas T, Niv Y, Malfertheiner P.. A network meta-analysis of randomized controlled trials on the treatment of eosinophilic esophagitis in adults and children. J Clin Gastroenterol. 2021;55(5):400–410. doi: 10.1097/MCG.0000000000001356. [DOI] [PubMed] [Google Scholar]
- 38.Tomizawa Y, Melek J, Komaki Y, et al. Efficacy of pharmacologic therapy for eosinophilic esophagitis: a systematic review and network meta-analysis. J Clin Gastroenterol. 2018;52(7):596–606. doi: 10.1097/MCG.0000000000000878. [DOI] [PubMed] [Google Scholar]
- 39.Visaggi P, Barberio B, Del Corso G, et al. Comparison of drugs for active eosinophilic oesophagitis: systematic review and network meta-analysis. Gut. 2023;72(11):2019–2030. doi: 10.1136/gutjnl-2023-329873. [DOI] [PubMed] [Google Scholar]
- 40.Lucendo AJ, López-Sánchez P.. Targeted therapies for eosinophilic gastrointestinal disorders. BioDrugs. 2020;34(4):477–493. doi: 10.1007/s40259-020-00427-w. [DOI] [PubMed] [Google Scholar]
- 41.Yanagibashi T, Satoh M, Nagai Y, et al. Allergic diseases: from bench to clinic – contribution of the discovery of interleukin-5. Cytokine. 2017;98:59–70. doi: 10.1016/j.cyto.2016.11.011. [DOI] [PubMed] [Google Scholar]
- 42.Nhu QM, Aceves SS.. Current state of biologics in treating eosinophilic esophagitis. Ann Allergy Asthma Immunol. 2023;130(1):15–20. doi: 10.1016/j.anai.2022.10.004. [DOI] [PubMed] [Google Scholar]
- 43.Avlas S, Shani G, Rhone N, et al. Epithelial cell-expressed type II IL-4 receptor mediates eosinophilic esophagitis. Allergy. 2023;78(2):464–476. doi: 10.1111/all.15510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pesek RD, Gupta SK.. Future therapies for eosinophilic gastrointestinal disorders. Ann Allergy Asthma Immunol. 2020;124(3):219–226. doi: 10.1016/j.anai.2019.11.018. [DOI] [PubMed] [Google Scholar]
- 45.Rothenberg ME, Dellon ES, Collins MH, et al. Efficacy and safety of dupilumab up to 52 weeks in adults and adolescents with eosinophilic oesophagitis (LIBERTY EoE TREET study): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Gastroenterol Hepatol. 2023;8(11):990–1004. doi: 10.1016/S2468-1253(23)00204-2. [DOI] [PubMed] [Google Scholar]
- 46.Youngblood BA, Brock EC, Leung J, et al. Siglec-8 antibody reduces eosinophils and mast cells in a transgenic mouse model of eosinophilic gastroenteritis. JCI Insight. 2019;4(19)doi: 10.1172/jci.insight.126219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lucendo AJ. Pharmacological treatments for eosinophilic esophagitis: current options and emerging therapies. Expert Rev Clin Immunol. 2020;16(1):63–77. doi: 10.1080/1744666X.2019.1705784. [DOI] [PubMed] [Google Scholar]
- 48.Ko E, Chehade M.. Biological therapies for eosinophilic esophagitis: where do we stand? Clin Rev Allergy Immunol. 2018;55(2):205–216. doi: 10.1007/s12016-018-8674-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The corresponding author is available with the study’s data upon reasonable request.